1 Introduction
The search for new final destinations for
residue from industrial processes has grown due to the interest of the
industries in searching for more viable alternatives in the economic and
environmental scope. Nowadays, it is known that the industries responsible for
the manufacture and transformation of materials produce, to a greater or lesser
degree, a certain amount of residue that is not always reused or has an ecologically
correct destination.
In the forestry sector, the generation of
residue has a significant impact, mainly for the pulp and paper industries,
which face environmental problems due to the significant generation of residue
from its production. According to Bellote et al. (1998), the generation of 48
tons of residue for every 100 tons of pulp produced makes it impossible to
choose landfills as final destination, due to the high maintenance and
implantation costs, as well as the need for handling with care recurring risks
of environmental contamination.
As an alternative to the landfill, the use
of industrial residue in agriculture has proved to be viable considering the
soil's potential to inactivate part of the chemical compounds present in such
residue, reducing its pollutant potential and, at the same time, the residue can
be used as low-cost agricultural inputs.
Residue from pulp and paper industries has
been highlighted when it comes to application to the soil and its consequences
for the various types of plantations. As evidenced by the studies carried out
in the area, the potential of the pulp and paper production residues is due to
the capacity to raise soil pH and to positively influence nutrient cycling,
functioning as a nutrient supply demanded by the crops (ALMEIDA et al. , 2008).
It is worth mentioning that the
characteristics of a residue generated by an industry depend directly on its
production process. In the pulp and paper sector, in general, the industrial
interest is the extraction of wood pulp, separating it from lignin, minerals
and resins, producing a pulp with high quality, with competitive prices and
with minimum environmental impact. There are different industrial methods that
realize the obtaining of cellulosic fibers, however it is highlighted as main
method the pulp kraft, which allows the obtaining of a pulp of high resistance
and with low lignin content, besides being distinguished for having as
principal the recovery of the chemicals used (FAVARO, 2015).
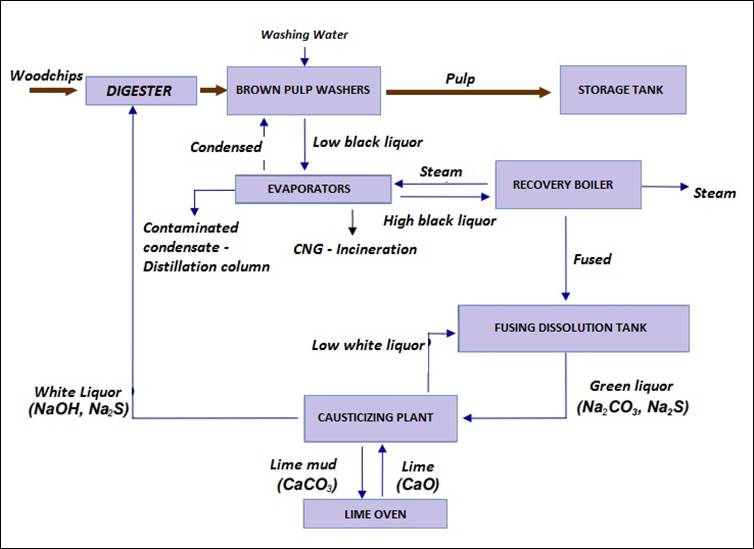
The pulping process using the kraft method
uses what is called a white liquor, formed of sodium hydroxide (NaOH) and sodium
sulfide (Na2S), for the digestion of wood lignin and the formation
of cellulose pulp. At the end of this stage, the black liquor is expelled from
the digester and has its composition based on: chemicals used, dissolved lignin
and carbohydrates. While pulp goes into the industrial process, it is necessary
that black liquor be recovered, due to economic and environmental issues.
Briefly, black liquor is withdrawn from the pulp in brown pulp washing systems
and then evaporated, so that it is concentrated, and its product is burned in
the recovery boilers. The solid residue of this stage is called dregs (FOELKEL,
2011).
The process characteristic with reuse
begins already in the boiler, where the steam produced is used in pulping and
papermaking, as well as for the production of electric energy for the factory.
After molten, the black liquor goes into a melt dissolution tank, forming the
green liquor which has as components sodium carbonate (Na2CO3)
and sodium sulfide (Na2S). Following the process occurs the
causticisation step, in which the sodium carbonate is placed in reaction with
lime (CaO) and gives rise to lime mud (CaCO3) and sodium hydroxide,
which can be reincorporated into the industrial process together with the
sodium sulfide to form the white liquor again (GOMIDE et al, 2006). The residue
from the preparation of hydrated lime, consisting mainly of lime mud is called
grits. The flow chart of the kraft recovery cycle can be seen in Figure 1.
Throughout the process, part of the
residues mentioned above and fluids from the production process generate
effluents that are conveyed to the effluent treatment plant. As is common in
effluent treatment processes, after the removal of the liquid portion of the
treated effluent, a semi-solid and pasty residue called effluent sludge is
generated. In pulp and paper industries, in addition to the residues mentioned
above, this sludge is also composed of a large concentration of cellulose which
makes it commonly called cellulose fiber, because of its physical and chemical
characteristics.
Figure 1 – Kraft
recovery cycle flowchart (GOMIDE et al, 2006)
Kraft pulp represents a large part of the
Brazilian pulp and paper sector, with South America being considered as the
largest producer of eucalypt kraft pulp in the world. This continent is
becoming the world's pole of production of eucalyptus pulp, so it is imperative
that South America lead research, innovation and development initiatives, not
only in the production and use of cellulosic pulp, but also in studies to the
reuse or treatment of solid wastes from their production, such as the aforementioned
disposal in the soil.
However, the disposal of residue of any
kind in the soil should not be carried out without first conducting studies in
order to take due account of possible changes in the physical and chemical
properties of the soil. Knowing the behavior of the material in the soil and
its implications is essential since the interactions with the medium result in
smaller or larger scale impacts to the biotic and abiotic environment.
Thus, with the objective of analyzing the
previous viability for the application of the cellulose fiber residue in the
soil, this study has as premise to carry out the classification of this residue
according to the norms and current legislation in order to classify the
hazardousness of the fiber of cellulose, determining whether there are
characteristics that make it a hazardous or non-hazardous, inert or non-inert
residue, as well as its behavior under solubilization and leaching tests.
2 Material and methods
The by-product assessed in this study
consists of residual fiber from cellulose manufacture. This recovered fiber are
residues from the industrial equipment, carried to the industrial wastewater
treatment plant. Since this residue is composed of a high concentration of
organic matter, it attracts attention to its use in the agriculture
The characterization of the residue of the
cellulose fiber, as the current legislation indicates, is an essential stage to
ensure the correct disposal and reuse of this residue. The chemical analysis
and classification were performed according to the recommended methodology
indicated by the Brazilian Association of Norms and Techniques (ABNT).
Therefore, to carry out the sampling, the
ABNT NBR 10007/2004 standard (ABNT, 2004a) was used, which determines the
standards for the sampling procedure of a representative amount of solid
residue, aiming to determine its characteristics regarding classification,
treatment methods, among others.
For the residue classification test, ABNT
NBR 10004/2004 (ABNT, 2004b) was used – Residue classification as to its
potential risks to the environment and public health, so that they can be
properly managed. This test analyses if the sample exhibits flammable,
corrosive, pathogenic and toxic characteristics.
The leaching test was carried out using the
ABNT NBR 10005/2004 standard (ABNT, 2004c) - Procedure for obtaining a leach
extract from solid residue that establishes the requisites required to obtain
this extract, in order to differentiate the residue classified by ABNT NBR
10004 /2004 as class I - hazardous - and class II - non - hazardous. The
leaching test was performed following the ABNT standards, in which a 100 g mass
of the sample was put in contact with 2000 mL of extraction solution 1 (5.7 mL
of glacial acetic acid, distilled and deionized water and 64.3 mL of 1.0 N NaOH,
made up to 1 L volume) for 18 ± 2 hours at 23 ± 2 ° C.
In the solubilization test, the reference
standard was ABNT NBR 10006/2004 (ABNT, 2004d) - Procedure to obtain a solubilized
solid residue extract, in order to differentiate the residues classified in
ABNT NBR 10.004 / 2004 as class II A - not inert - and class II B - inert. The
solubilization test was carried out according to the ABNT procedures, in which
a 250 g mass of the sample was put in contact with 1000mL of deionized water
for 7 days at a 23 ± 2 ºC temperature.
3 Results and discussion
The cellulose fiber characterization following
the ABNT NBR 10004/2004 standard showed that the residue did not present
flammability, reactivity, corrosivity, toxicity and pathogenicity
characteristics according to the terms considered by the standard. Therefore,
the sample was classified as non-hazardous (Class II). In terms of reactivity,
the presence of sulfide must be evaluated and the residue must present lower
than 500 mg of releasable H2S according to the United States
Environmental Protection Agency (USEPA) – SW 846 test methods, for the solid
waste evaluation test (USEPA, 2015). In our study we did not identified the
presence of sulfide.
It is important to mention that the
evaluation of sulfide in cellulosic fiber is of importance. Its occasional
presence in an residue is associated to the use of sulfur products in the
composition of the digestion liquor, causing secondary reactions with some
organic components that are present in the raw material, giving rise to the
organosulfides, gaseous materials that have an unpleasant odor. Besides the
hydrogen sulfide (H2S), other compounds are also considered organosulfurized
such as: methyl mercaptan (CH3SH), dimethyl sulfide [(CH3)2S]
and dimethyl disulfide [(CH3)2S2]. These
compounds make up a set called TRS (Total Reduced Sulfur) or reduced sulfur
compounds (MISHAL, 1975). These compounds are considered to be severe air
pollutants even at parts per billion (ppb) levels (OSSES, 1991). So, this
pollutant must be considered in studies involving waste reuse.
The determination of the transferability of
organic and inorganic substances present in the residues was carried out using
the leaching test determined by the ABNT NBR 10005/2004 standard, resulting in
a leached solution with pH of 5.47 after the contact period. For the results of
the leaching test, it can be inferred that none of the analyzed parameters are
above the maximum permitted value (MPV), conferring non-toxicity
characteristics, in the referred terms of the standard.
The solubilization test in the terms of the
ABNT NBR 10006/2004 standard, has the objective of identifying the
concentration of water-soluble substances. The results obtained for the
solubilization test are set out in the Table 1.
Table 1 – Determination
values of the solubilization test
|
Solubilization
Parameters
|
Obtained value
|
Unit
|
MPV
|
|
Aldrin + Dieldrin
|
<0.0003
|
mg/L
|
0.00003
|
|
Aluminum
|
0.412
|
mg/L
|
0.2
|
|
Arsenic
|
<0.002
|
mg/L
|
0.01
|
|
Barium
|
0.72
|
mg/L
|
0.7
|
|
Cadmium
|
0.022
|
mg/L
|
0.005
|
|
Lead
|
<0.002
|
mg/L
|
0.01
|
|
Cyanide
|
<0.005
|
mg/L
|
0.07
|
|
Chlordane
|
<0.0002
|
mg/L
|
0.0002
|
|
Chloride
|
7.023
|
mg/L
|
250
|
|
Copper
|
<0.006
|
mg/L
|
2
|
|
Chrome
|
<0.005
|
mg/L
|
0.05
|
|
2.4-D
|
<0.03
|
mg/L
|
0.03
|
|
DDT+DDD+DDE
|
<0.002
|
mg/L
|
0.002
|
|
Endrin
|
<0.0004
|
mg/L
|
0.0004
|
|
Phenols
|
0.74
|
mg/L
|
0.01
|
|
Iron
|
96.36
|
mg/L
|
0.3
|
|
Fluoride
|
<0.05
|
mg/L
|
1.5
|
|
Heptachlor Expoxide
|
<000003
|
mg/L
|
0.00003
|
|
Hexachlorobenzene
|
<0.001
|
mg/L
|
0.001
|
|
Lindane
|
<0.002
|
mg/L
|
0.002
|
|
Manganese
|
21.88
|
mg/L
|
0.1
|
|
Mercury
|
<0.0002
|
mg/L
|
0.001
|
|
Methoxychlor
|
<0.02
|
mg/L
|
0.02
|
|
Nitrate
|
0.335
|
mg/L
|
10
|
|
Silver
|
<0.002
|
mg/L
|
0.05
|
|
Selenium
|
<0.002
|
mg/L
|
0.01
|
|
Sodium
|
209.59
|
mg/L
|
200
|
|
Sulfate
|
149.775
|
mg/L
|
250
|
|
Surfactants
|
<0.10
|
mg/L
|
0.5
|
|
Toxaphene
|
<0.005
|
mg/L
|
0.005
|
|
2.4.5-T
|
<0.002
|
mg/L
|
0.002
|
|
2.4.5-TP
|
<0.03
|
mg/L
|
0.03
|
|
Zinc
|
0.192
|
mg/L
|
5
|
|
pH – Solubilized
|
6.45
|
-
|
-
|
*The lines highlighted in bold show the parameters
analyzed that extrapolated the maximum value by the ABNT NBR 10006/2004
standard
From the results obtained for the
solubilization test, the values found above the maximum permitted value for the
manganese, aluminum, barium, cadmium, phenols, iron, and sodium are
highlighted. This shows that the cellulose residue used in the study does not
present inert characteristics, that is, it demonstrates characteristics of
solubilization in water for some of its components, that way presenting a
potential of degradation or contamination of the water or the atmosphere.
Therefore, the waste is classified as class IIA (non-inert and non-hazardous)
The cadmium (Cd) concentration above the allowed
value may be related to the industrial machinery since this element is applied
for the steel and iron covering due to its high resistance to corrosion. It is
known that in the kraft process, the cadmium is found in low concentrations in
the raw materials and are removed by the liquor filtration system and by the
removal of dregs and grits (FOEKEL, 2011). The bioaccumulation potential of
cadmium in aquatic plants, invertebrates, fish, and mammals makes this an
element to be closely monitored, requiring attention to its final disposal.
The presence of phenols is often associated
by the pulping and papermaking process in the industry, due to the black
liquor, an effluent with high organic load generated in the kraft pulp process,
which is responsible for the removal of approximately 90% of the lignin present
in the wood (PERALTA-ZAMORA et al, 1997). The phenols determination before any
disposal of the residue is essential since this compound might be considered
toxic and potentially carcinogenic (SANTANA et al, 2009) and may affect the
taste and odor of drinking water even in low concentrations. At high
concentrations, phenolic compounds have destructive potential on aquatic fauna
and flora, due to its toxic characteristics and the high demand for oxygen.
For the pulp and paper industry, the
presence of manganese (Mn) is seen as a factor to be avoided in the production
process since, when mixed with iron (Fe) and copper (Cu), it causes negative
action on hydrogen peroxide stability, the substance used in acid washing for
cellulose bleaching. Manganese and iron can cause the catalytic decomposition
of hydrogen peroxide, generating hydroxyl radicals that attack and damage the
cellulose structure, reducing its physical properties (SIQUEIRA, SILVA FILHO
& SECCOMBRE, 2012).
The industries adopt chemical processes to
reduce the iron and manganese contents, so it is believed that the high values
recorded for these two parameters are related to their removal in the
production process, to their presence in the wood , water or even to their
generation by the equipment of the factory, causing them to be carried to the
treatment plant and consequent residue. For soil application, its known that
manganese is a nutrient and a limiting factor in the interaction with plants.
One of the main problems for soybean cultivation, for example, is Mn-deficient
soils (ROSOLEM et al., 1992). However, establishing critical levels of
manganese in the soil becomes difficult, since its soil determination is
strongly influenced by pH, and it is necessary to accomplish more specific
studies for the application of various manganese dosages on different pH
values.
For the aluminum levels (Al) observed, it
is known that the major inputs of this element in the kraft process industries
occur by the industrial water, by treatment using aluminum sulfate as a
flocculant, and by the limestone or lime used for the calcium replacement in the
lime system. Although most of it is precipitated by the recovery system and
collected by the green liquor filtration system (FOEKEL, 2011), some of the
aluminum may not be recovered and can be carried to the final residue of the
industrial process.
The special attention given to aluminum is
due to the fact that it is a neurotoxic compound with potential to cause damage
to human health when found in high concentrations. In the soil application,
studies report that although Al is not considered an essential element for
plant species, the application in low concentrations can stimulate the growth
of some plants, but on the other hand, if not applied in the correct
concentrations for each species, can cause cytological abnormalities in plants,
retarding the root growth (FERREIRA, MOREIRA & RASSINI, 2006).
The observed presence of sodium (Na) in the
solubilized has its main origin due to the use of compounds of this element in
the industrial process of lignin digestion and bleaching of the cellulosic
pulp. Although the kraft process presents low losses of sodium and sulfate due
to the mechanisms of recovery, it is estimated that the losses of these
elements are between 5 and 10%, which may justify the presence in the final
residue (FOELKEL, 2011).
A possible increase in the sodium
concentration in the soil due to the disposal of the residue shall be observed
with caution and demonstrates the necessity of monitoring, since with the
increase of the exchangeable Na+ content in the soil, some important
properties of the soil can be impaired from the agronomic point of view, such
as clay and organic substances dispersion (ALMEIDA et al, 2007). Another study
carried out with the disposal of residues with high sodium content in sandy
soils showed a higher susceptibility of the residues to the leaching of sodium
in the soil profile and could contaminate the groundwater (TRIGUEIRO, 2006).
The occurrence of barium (Ba) in pulp and
paper industries comes almost exclusively from wood and tree bark. Barium is an
element that demands special attention for the industries since its
precipitation as barium sulphate is one of the most undesirable in the fiber
line. This is due to the low solubility of the barium precipitate, making it
difficult to be removed mechanically or chemically, being a potential factor of
incrustations in the machinery of the industry (FOELKEL, 2011). Thus, the
precipitate is removed with the combination of mechanical treatments with
strong water jets, chelating agents, and acid washing, which can then carry concentrations
of this element up to the final residue of the production.
Although it is found naturally in the soil,
the high levels of barium can be toxic to plants and invertebrates, also
interfering with the sulfur availability, due to the formation of low
solubility sulfates (KUPERMAN et al, 2006). As for human health, it is known
that only 2% of ingested barium is absorbed by the body, tending to accumulate
in the bones, replacing calcium.
4 Final Considerations
The results obtained by the tests infer
that the residual fiber of pulp and paper industry presented values that do not
confer inert characteristics and classifies it as non-hazardous Residue Class
IIA. It is worth mentioning that the present study had the objective to carry
out the classification of the solid residue in question, to analyze and to seek
explanations for the values obtained, in order to characterize the residual
fiber and to serve as an input for the process of decision making on the
applicability of the residue in the soil.
Acknowledgements
The authors of this paper would like to
thank the support for this project provided by Universidade do Estado de Santa
Catarina (UDESC), Universidade do Oeste de Santa Catarina (UNOESC) and the
Laboratory of Water and Waste Treatment of CAV / UDESC.
References
ALMEIDA, H.C. et al. Influência da adição de um resíduo alcalino da Indústria de
papel e celulose na lixiviação de cátions em um solo ácido. Revista Brasileira de Ciência do
Solo. 2008; v32, n4, p.1775-1784.
ALMEIDA, H.C. et al. Composição
química de um resíduo alcalino da indústria de papel e celulose (Dregs). Química
Nova. 2007; v30, n7, p.1669-1672.
Associação
brasileira de normas técnicas. NBR 10007: Amostragem de resíduos sólidos.
Rio de Janeiro (Brazil): ABNT, 2004.
Associação
brasileira de normas técnicas. NBR 10004: Resíduos sólidos - classificação.
Rio de Janeiro (Brazil): ABNT, 2004.
Associação
brasileira de normas técnicas. NBR 10005: Procedimento para obtenção de
extrato lixiviado de resíduos sólidos. Rio de Janeiro (Brazil): ABNT, 2004.
Associação
brasileira de normas técnicas. NBR 10006: Procedimento para obtenção de
extrato solubilizado de resíduos sólidos. Rio de Janeiro (Brazil): ABNT, 2004.
BELLOTE,
A.F.J. et al. Resíduos
da indústria de celulose em plantios florestais. Colombo. 1998:
1-8.
FAVARO,
J.S.C. Estudos da polpação kraft, branqueamento de refino de Eucalyptus
grandis x Eucalyptus urophylla. [thesis].
Guaratinguetá: Curso de Engenharia Mecânica, Faculdade de Engenharia de
Guaratinguetá/Universidade Estadual Paulista; 2015. 180 f.
FERREIRA,
R.P.; MOREIRA, A; RASSINI, JB. Toxidez de alumínio em culturas anuais. Embrapa
Pecuária Sudeste-Documentos (INFOTECA-E), 2006.
FOELKEL,
C. Os eucaliptos e os elementos não processuais na fabricação de celulose
kraft. Eucalyptus
Online Book & Newsletter [Internet]. 2011. Available from: http://www.
eucalyptus. com. br/eucaliptos/PT24_ElementosNproces. pdf
GOMIDE, J.L. et al. Tecnologia e Química da Produção de Celulose. Viçosa:
Laboratório de Celulose e Papel, Universidade Federal de Viçosa; 2006: 235 p
KUPERMAN, R.G. et al. Toxicity benchmarks for antimony, barium, and beryllium determined
using reproduction endpoints for Folsomia candida, Eisenia fetida, and
Enchytraeus crypticus. Environmental toxicology and chemistry. 2006; v35, n3,
p.754-762.
MISHAL, B.T. Kraft pulping
and atmospheric gaseous emissions. IPTTA Souvenir. 1975: 95-103.
OSSES, M. Las emisiones de olores de una
planta de celulosa kraft. Celulosa y Papel. 1991: 6-16.
PERALTA-ZAMORA,
P. et al. Remediação de efluentes derivados da indústria de papel e celulose. Tratamento
biológico e fotocatalítico. Química Nova. 1997; 20 (2): 186-190.
ROSOLEM, C.A. et al. Manganês no solo, sua avaliação e toxidez de manganês em
soja. Pesquisa Agropecuária Brasileira. 1992; v27, n2, p. 277-285.
SANTANA, C.M. et al. Methodologies
for the Extraction of Phenolic Compounds from Environmental Samples: New Approaches. Molecules. 2009; 14: 298-320.
SIQUEIRA,
J.L.D; SILVA FILHO, LL.; SECCOMBE, R. Branqueamento de polpa kraft de
eucalipto-o papel do peróxido de hidrogênio. Peróxidos do Brasil
Ltda–São Paulo–SP, 2012.
TRIGUEIRO R.M. Efeito de “dregs e grits”
nos atributos de um neossolo quartzarênico e na produção volumétrica de
eucalipto [thesis]. Botucatu: Universidade Estadual
Paulista; 2006. 73 p.
USEPA, Environmental
Protection Agency. SW-846: Test Methods for Evaluating Solid Waste,
Physical/Chemical Methods. Washington (USA): USEPA, 2015.