1 Introduction
Meeting the demand
for good quality water for cities is one of the major current challenges.
Population growth, food production and industrial development can lead to
serious problems in water supply both in increasing demand for consumption and
in contamination of bodies of water. Agriculture consumes around 70% of the
world's water resources, followed by the industrial sector with 20% and the
domestic sector with 10%. These values may vary according to the country's
development (UNESCO,
2014).
Ensuring the
availability and sustainable management of water and sanitation for all is one
of the 17 sustainable development goals adopted in the General Resolution of
the United Nations in September 2015. This resolution known as Agenda 2030 in
its objective 6, subitem 6.3, mentions improving water quality, reducing
pollution, eliminating eviction and minimizing the release of hazardous
chemicals and materials (United Nations, 2015).
The use of
agrochemicals in agriculture has been intensified in recent decades with the
aim of increasing food production; on the other hand, these pesticides can be sources
of contamination for rivers and groundwater and, because of their intrinsic
nature, it can cause damages to human health that vary according to the active
principle and the absorbed dose (BRASIL,
2016).
Among the
pesticides, glyphosate is the most sold in the world. In Brazil alone,
glyphosate represents 40% of sales of pesticides. Glyphosate is an
organophosphorus, non-selective, systemic and post-emergent herbicide used in
many food and non-food crops, as well as non-agricultural areas, such as
recreational areas (WEIDENHAMER and CALLAWAY, 2010). Its physicochemical
properties are relatively low molecular weight, high polarity, high water
solubility, low solubility in organic solvents, amphoteric behavior, and easy
formation of metal complexes (SANCHIS et al., 2012).
In its early years,
it was believed that this herbicide, did not cause damage to human health but
with the intensification of its application in crops due to the development of
seeds resistant to this herbicide, new studies have pointed out the contrary (CARNEIRO
et al., 2015). One study showed that initial toxicity
tests indicated that glyphosate had low toxicity risks, prompting regulatory
authorities around the world to allow for high levels of exposure to
glyphosate; however, studies published in the last decade point to the need for
a new look at the toxicity of glyphosate. In addition, the authors state that
the International Agency for Research on Cancer, linked to the World Health
Organization, has recently concluded that glyphosate is "probably
carcinogenic to humans" (MYERS
et al., 2016; TARONE, 2018).
Other studies also
claim that glyphosate poses risks to human or other mammalian health such as: acute
and chronic neurotoxicity in rats(CATTANI
et al., 2014), neurodegenerative diseases such as
Parkinson's disease (GUI
et al., 2012) and endocrine problems in rats (CLAIR
et al., 2012).
The persistence and
transport of glyphosate in the soil are dependent on soil composition, climatic
conditions and microbiological activity, as well as agricultural management
(GIMSING et al., 2004). Most of the literature reports biological impacts of
glyphosate based on laboratory tests or short-term field studies (HELANDER et
al., 2012).
In addition, due to
the wide variety of application methods and climatic conditions, a significant
amount of glyphosate reaches the soil and may be leached from the root region
in drainage water and groundwater. Experimental observations combined with
transport studies suggest that the transport of glyphosate may occur due to
heavy rains shortly after application in moist soils leading to the formation
of preferential pathways flows (DAMONTE et al., 2007).
In this context,
one of the promising technologies for the removal of pollutants from water is
adsorption since it is a low cost and high efficiency method. Carbon-based
materials, such as graphene, have been extensively studied in adsorption
applications to remove contaminants from water due to their properties (CHOWDHURY
et al., 2014). One of the difficulties in the use of
adsorbent materials is its removal after the adsorption process. The
association of graphene with magnetic materials may facilitate such removal
work because the adsorbent material may be retained by a magnetic field without
the need for filtration or centrifugation (WANG
et al., 2015).
In comparison with
other materials formed by carbon, graphene has attracted the attention of
researchers due to its properties of an efficient association with metals and
metal oxides forming hybrids; this is owing to its properties of high
electrical conductivity, mechanical resistance and large specific area. Yao et
al. (2014) used a MnFe2O4 graphene hybrid as catalysts in
the removal of organic pollutants from water. Kumar et al. (2014) also used a
graphene oxide hybrid (MnFe2O4) in the removal of lead
and arsenic from contaminated water. The authors considered that the easy
magnetic separation, high removal efficiency, large surface area and the fact
of being reusable are factors that make this hybrid a strong candidate to be
used as a low cost adsorbent in the removal of toxic metals from water.
Specifically for
the removal of glyphosate from water, in a previous work it was synthesized,
characterized and tested the MnFe2O4-G composite
succeeding in removing this contaminant from water (YAMAGUCHI
et al., 2016). Although there are several recent studies
using magnetic graphene for water treatment, it was noticed that an industrial
application was not aimed, and it was used batch scale laboratorial assays with
different equipment for each step of the processes.
This whole scenario
motivated a previous research for the development of a prototype in a pilot
scale using the MnFe2O4-G composite. The results were
satisfactory, the prototype was evaluated in relation to the removal of
glyphosate in four complete cycles of adsorption and desorption, obtaining a
84.5% removal of glyphosate in the first cycle, 80% in the second and third
cycle and 60% in the fourth cycle (SANTOS et al., 2017).
Therefore, the
proposal addressed in the present research had as objective the design and
construction of a single prototype that gather all the steps of water
decontamination with glyphosate using a hybrid compound of MnFe2O4-G,
aiming to provide a concept for applications in water treatment plants. Thus,
it was focused the determination of the steps to glyphosate removal, the
definition of the layout, the selection of materials, the project elaboration,
the construction of the prototype in a reduced scale.
2 Materials and methods
2.1
Determination of the steps for the removal of glyphosate using hybrid manganese
ferrite compound and graphene
Initially, the
volume of 1 dm3 of water was assigned to be treated. After that, it
was necessary to determine the steps for the treatment of water contaminated
with glyphosate, in order to design the prototype. For that, it was used the
work developed by Yamaguchi et al. (2016) and Santos et al. (2017) for the
determination of these steps and analysis methods.
The first step of
the process was to place 1.0 g of particles in contact with glyphosate
contaminated water (20 ppm) and stirred them for a period of 5 hours in order
to perform the adsorption. In this way, the first part of the prototype worked
with a tank coupled with a stirrer. This step was called the adsorption step.
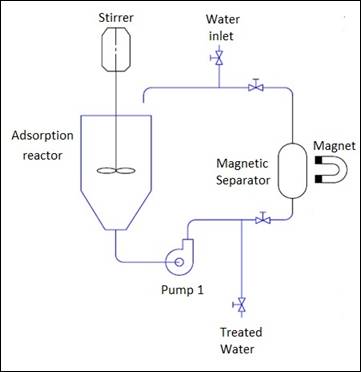
After the
adsorption step, the particles had to pass through a magnetic field to be
separated from the water that was decontaminated. A magnetic separator
connected in parallel with the adsorption reactor was designed as shown in
Figure 1. In that separator, a magnetic field was approached or moved away from
the walls of the reservoir according to the need for separation. The flow
velocity was reduced and the water was pumped from the adsorption reactor to
the magnetic separator and returned to the adsorption tank. As a result, the
water passed several times through the magnetic field for a better retention of
the particles. In order to measure the efficiency of the magnetic separator,
the MnFe2O4-G particles were weighed at the beginning of
the experiment and at the end of all 4 cycles. This
step was called magnetic separation.
Figure 1 - Flow diagram of the
adsorption reactor and magnetic separator
After the magnetic
separation, the decontaminated water was drained and discarded. In an
industrial-scale situation, this water could be referred to other treatments
according to the necessity.
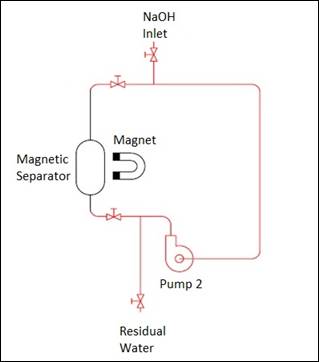
After this first
magnetic separation, the particles contaminated by glyphosate were retained in
the separator and must undergo a desorption process so that the contaminant is
removed from the particles, allowing them to be reused in new cycles of adsorption.
In the desorption process, the particles were contacted with a solution of 0.1
M NaOH and stirred for a period of time empirically defined in 15 minutes. For
this purpose, the same magnetic separator reservoir was used, this time without
the magnetic field, and a pump was used as a stirrer, as shown in Figure 2. The
desorption was completed and the particles were again magnetically
Figure 2 - Flow diagram of the
desorption system
After desorption,
these particles were rinsed with pure or distilled water to remove the excess
of NaOH to which the particle had been exposed. This rinsing was performed in
the same manner as in the desorption using the magnetic separator resevoir
without the field and a 10 minute empirically assigned time bomb.
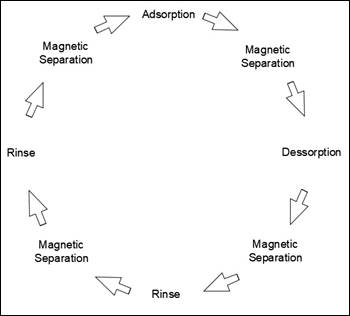
The rinsing step
may be performed more than once to ensure maximum removal of NaOH. After each
rinsing step, a new magnetic separation was performed, the rinse water was
discarded, and the MnFe2O4-G particles were ready for
further treatment processes. This stage was called rinsing.
All steps were
cited and were performed in a predetermined order, according to the flowchart
shown in Figure 3.
Figure 3 - Flowchart with all
treatment steps
2.2
Definition of materials
The first
components to be acquired were the pumps. For this, since the prototype should
treat the volume of 1 dm3 of water, it was attributed that the pump
to be selected should have a flow rate of around 5 dm3min-1.
For a better control of the flow, the pump should be fed by direct current of
electricity; therefore, by varying the voltage of the pump, it would be possible
to vary the flow rate.
The adsorption
reactors and magnetic separator were made from PET bottles, with a volume of
1.5 dm3 for the adsorption reactor and 0.1 dm3 for the
magnetic separator. The hoses used were of silicone, 10 mm and transparent.
The magnets
selected for the magnetic separator were neodymium, type N52, with dimensions
of 40x20x10 mm.
To facilitate the
assembly, 10 mm pneumatic connections have been chosen so that the hoses could
be fastened and de-attached quickly like a quick coupling. Mass and epoxy resin
were used to fix the connections in the reservoirs.
3 Results and discussion
3.1
Prototype design
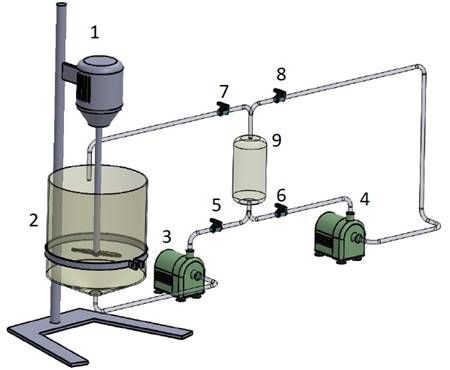
Figure 4 shows the layout defined for the construction of the prototype.
Note that the two systems that make up the removal process are present and
interconnected. On the left side of the layout is the glyphosate adsorption
system and on the right side, the particle regeneration system (desorption).
This was the initial proposition for setting up the prototype. Nonetheless, the
adsorption and desorption processes were not constructed interconnected in this
first moment. They were constructed separately but the interconnection can be
easily accomplished by means of valves as shown in Figure 4.
Figure 4 - Prototype and its components:
1 stirrer; 2 adsorption reactor; 3 and 4 pumps; 5, 6, 7 and 8 valves; 9
magnetic separator
3.2
Construction of the prototype
In Figures 5 and 6, it is possible to observe the constructed prototype
and being tested in the laboratory. Figure 5 shows the layout with the
configuration projected next to the built prototype, showing the adsorption
step. It also shows that the propeller stirrer was not required inside the
adsorption reactor, due to the fact that the selected and installed pump performed
the agitation of the particles successfully. Another modification concerns the
entry of water into the system, which in the initial layout should be performed
by a pipe. The water inflow into the system was carried out directly over the
upper inlet of the reactor, by manually supplying 1 dm3 of
artificially contaminated water with 20 ppm of glyphosate.
Figure 5 - Original layout alongside
the built prototype with the steps of adsorption and magnetic separation

It is further noted that the output of the treated water, which in the
original layout would be between the pump and the magnetic separator by means
of piping, occurred just before the magnetic separator by simply disconnecting
the hose before entering the separator. It is suggested, for later studies,
that all components of the original layout are connected, except the agitator
which proved to be unnecessary, just to make the system configure itself with a
better presentation. In terms of removal efficiency, it should be noted that the
simplifications adopted did not compromise the results.
Figure 6 shows the layout with the configuration designed next to the
built prototype, showing the desorption stage of the particles. As previously
explained, the entry of NaOH into the system and the exit of the waste water,
which in the initial layout should be performed by a suitable piping, occurred
just before the magnetic separator by simply disconnecting the hose.
Figure
6 - Original layout next to the prototype built with the adsorption and magnetic
separation steps

After the construction of the prototype, some tests of its operation were
carried out in order to identify possible leaks and other problems not foreseen
in the project.
3.3
Prototype test
A test was performed to verify the prototype's functioning and correction
of possible problems such as leaks. Regarding the magnetic separation, it was
found empirically that within 15 minutes the separation was completed and 70%
of the particles were recovered after 4 complete cycles of treatment, while 30%
of the particles were lost in the treated water in the regeneration NaOH
solutions, in the waters of rinsing and part were deposited in the walls of the
tanks and pipes.
The feasibility of use and regeneration of the material in the prototype was
also evaluated in an earlier study (SANTOS et al., 2017). The results showed that the treatment is
promising, since there was significant water decontamination in four complete
cycles, with 84.5% of glyphosate removal in the first cycle, 80% in the second
and third cycle and 60% in the fourth cycle.
A previous work (YAMAGUCHI et al., 2016) has also studied the magnetic
graphene composite, MnFe2O4–G. The material was was
characterized using various techniques, and then applied to remove glyphosate
from water in batch scale experiments. The results showed that the hybrid
composite presented a BET area of 305.5 m2/g and was well dispersed
on graphene nanosheets. It was also proposed that the adsorption mechanism of
glyphosate on MnFe2O4–G was through a combination of an
electrostatic interaction with an ion exchange that was favoured at low
temperature. The maximum adsorption capacity of glyphosate adsorption was 39 mg
g-1 at 5°C. The kinetics and isotherm data obtained fitted well with
pseudo-second-order kinetics and the Freundlich isotherm model, indicating a
multilayer chemisorption in a heterogeneous surface. It was also determined that
the adsorption of glyphosate on the MnFe2O4–G was spontaneous
and exothermic.
There are few studies of glyphosate removal from aquous solutions in the
literature. Some works studied the adsorption of glyphosate herbicide on
activated carbon and biochar. Mohsen et al. (2010) obtained a maximum
adsorption capacity of 48.4 mg g-1 in activated carbon derived from
a newspaper industry waste. Mayakaduwa et al. (2015) determined the kinetic and
equilibrium mechanisms for the adsorption of glyphosate by biochar and obtained
a maximum adsorption capacity of 44 mg g-1. Both results were
slightly higher when compared with the maximium adsorption capacity of MnFe2O4–G
(39 mg g-1) (YAMAGUCHI et al., 2016). However, the advantage of MnFe2O4–G
is its application in a prototype presenting all the steps of adsorption and
desorption for glyphosate decontamination in the same equipment using its
capability of reusability and magnetic separation.
4 Conclusion
The prototype was
conceived and designed with its components, materials and layout defined. After
constructing the prototype, it was possible to achieve the goal of bringing all
the treatment steps together into a single device, although at the moment it has
been built in two parts separately, which can be solved with the use of valves.
The tests were performed according to what was proposed and no operational
problems were identified. Although the recovery of the particles in the
magnetic separator has been satisfactory, improvements in this separation can
be developed in other studies. Additional tests may be performed for future
work for better understanding of the process, such as pH, replicates,
optimization of stirring speed, adsorption and desorption times, costs of the
processes, reusability of the adsorbent, use of different contaminants and the
use of real wastewaters. Also full-scale studies are recommended in order to
apply the concepts in a water treatment plant.
References
BRASIL. Relatório
Nacional de Vigilância em Saúde de Populações Expostas a Agrotóxicos.
Brasilia: Ministério da Saúde, p.141, 2016
CARNEIRO,
F. F.; AUGUSTO, L. G. D. S.; RIGOTTO, R. M.; FRIEDRICH, K.; BÚRIGO, A. C. Um
alerta sobre os impactos dos agrotóxicos na saúde. Rio de
Janeiro/São Paulo: EPSJV Editora Expressão Popular 2015.
CATTANI,
D.; CAVALLI, V.; RIEG, C. E. H.; DOMINGUES, J. T.; DAL-CIM, T.; TASCA, C. I.;
SILVA, F.; ZAMONER, A. Mechanisms underlying the neurotoxicity induced by
glyphosate-based herbicide in immature rat hippocampus: Involvement of
glutamate excitotoxicity. Toxicology, v.
320, n., p. 34-45, 2014.
CHOWDHURY,
S.; BALASUBRAMANIAN, R. Recent advances in the use of graphene-family
nanoadsorbents for removal of toxic pollutants from wastewater. Advances in
Colloid and Interface Science, v. 204, n. Supplement C, p. 35-56, 2014.
CLAIR,
É.; MESNAGE, R.; TRAVERT, C.; SÉRALINI, G.-É. A glyphosate-based herbicide
induces necrosis and apoptosis in mature rat testicular cells in vitro, and
testosterone decrease at lower levels. Toxicology in Vitro, v. 26, n. 2,
p. 269-279, 2012.
DAMONTE,
M.; TORRES SÁNCHEZ, R. M.; DOS SANTOS AFONSO, M. Some aspects of the glyphosate
adsorption on montmorillonite and its calcined form. Applied Clay Science,
v. 36, n. 1–3, p. 86-94, 2007.
GIMSING,
A. L.; BORGGAARD, O. K.; BANG, M. Influence of soil composition on adsorption
of glyphosate and phosphate by contrasting Danish surface soils. European
Journal of Soil Science, v. 55, n. 1, p. 183-191, 2004.
GUI,
Y.-X.; FAN, X.-N.; WANG, H.-M.; WANG, G.; CHEN, S.-D. Glyphosate induced cell
death through apoptotic and autophagic mechanisms. Neurotoxicology and
Teratology, v. 34, n. 3, p. 344-349, 2012.
HELANDER,
M.; SALONIEMI, I.; SAIKKONEN, K. Glyphosate in northern ecosystems. Trends
in Plant Science, v. 17, n. 10, p. 569-574, 2012.
KUMAR,
S.; NAIR, R. R.; PILLAI, P. B.; GUPTA, S. N.; IYENGAR, M. A. R.; SOOD, A. K.
Graphene Oxide–MnFe2O4 Magnetic Nanohybrids for Efficient Removal of Lead and
Arsenic from Water. ACS Applied Materials & Interfaces, v. 6, n. 20,
p. 17426-17436, 2014.
MAYAKADUWA,
S. S.; KUMARATHILAKA, P.; HERATH, I.; AHMAD, M.; AL-WABEL, M.; OK, Y. S.;
USMAN, A.; ABDULJABBAR, A.; VITHANAGE, M. Equilibrium and kinetic mechanisms of
woody biochar on aqueous glyphosate removal. Chemosphere, v., n., p.,
2015.
MOHSEN
NOUROUZI, M.; CHUAH, T. G.; CHOONG, T. S. Y. Adsorption of glyphosate onto
activated carbon derived from waste newspaper. Desalination and Water
Treatment, v. 24, n. 1-3, p. 321-326, 2010.
MYERS,
J. P.; ANTONIOU, M. N.; BLUMBERG, B.; CARROLL, L.; COLBORN, T.; EVERETT, L. G.;
HANSEN, M.; LANDRIGAN, P. J.; LANPHEAR, B. P.; MESNAGE, R.; VANDENBERG, L. N.;
VOM SAAL, F. S.; WELSHONS, W. V.; BENBROOK, C. M. Concerns over use of
glyphosate-based herbicides and risks associated with exposures: a consensus
statement. Environmental Health, v. 15, n., p. 13, 2016.
SANCHIS,
J.; KANTIANI, L.; LLORCA, M.; RUBIO, F.; GINEBREDA, A.; FRAILE, J.; GARRIDO,
T.; FARRE, M. Determination of glyphosate in groundwater samples using an
ultrasensitive immunoassay and confirmation by on-line solid-phase extraction
followed by liquid chromatography coupled to tandem mass spectrometry. Anal
Bioanal Chem, v. 402, n. 7, p. 2335-2345, 2012.
SANTOS,
J. C. M.; SOUSA, J. C. A.; ALMEIDA, A. C. S.; HOMEM, N. C.; BERGAMASCO, R.;
GASPAROTTO, F.; REZENDE, L. C. S. H.; YAMAGUCHI, N. U. Pilot Batch-Scale
Reactor for Glyphosate Removal Using Hybrid Magnetic Graphene. Chemical
Engineering Transactions, v. 60, n., p. 6, 2017.
TARONE,
R. E. On the International Agency for Research on Cancer classification of glyphosate
as a probable human carcinogen. European Journal of Cancer Prevention,
v. 27, n. 1, p. 82-87, 2018.
UNESCO.
The United Nations World Water Development Report 2014: Water and
Energy. Paris: 2014
UNITED
NATIONS. Transforming our world: the 2030 Agenda for Sustainable
Development. 2015.
Disponível em
http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E. Acesso
em 08/03/2018.
WANG,
H.; YUAN, X.; WU, Y.; CHEN, X.; LENG, L.; WANG, H.; LI, H.; ZENG, G. Facile
synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic
composites and its application for the Cr(VI) removal. Chemical Engineering
Journal, v. 262, n. Supplement C, p. 597-606, 2015.
WEIDENHAMER,
J. D.; CALLAWAY, R. M. Direct and indirect effects of invasive plants on soil
chemistry and ecosystem function. J Chem Ecol, v. 36, n. 1, p. 59-69,
2010.
YAMAGUCHI,
N. U.; BERGAMASCO, R.; HAMOUDI, S. Magnetic MnFe2O4–graphene hybrid composite
for efficient removal of glyphosate from water. Chemical Engineering Journal,
v. 295, n. Supplement C, p. 391-402, 2016.
YAO,
Y.; CAI, Y.; LU, F.; WEI, F.; WANG, X.; WANG, S. Magnetic recoverable MnFe2O4
and MnFe2O4-graphene hybrid as heterogeneous catalysts of peroxymonosulfate
activation for efficient degradation of aqueous organic pollutants. Journal of
Hazardous Materials,
v. 270, n. Supplement C, p. 61-70, 2014.