Ci. e Nat., Santa Maria v.42, e27, 2020
DOI:10.5902/2179460X41226
ISSN 2179-460X
Received 19/11/19 Accepted:
15/01/20 Published:24/06/20

Environment
Scientometric
analysis applied to the water treatment with activated carbon
Análise cientométrica aplicada ao tratamento de água com carvão ativado
Kelly Kathleen Almeida HeylmannI
Bruno
Vasconcellos LopesI
Carolina
Faccio DemarcoIII
Thays
França AfonsoIV
Tito
Roberto Sant'Anna Cadaval JúniorV
Andrei
Vallerão IgansiVI
Maurizio
Silveira QuadroVII
Robson AndreazzaVIII
I Universidade Federal de Pelotas, RS, Brasil -
kellyheylmann@hotmail.com
II Universidade
Federal de Pelotas, RS, Brasil -lopesbruno13@gmail.com
III Universidade
Federal de Pelotas, RS, Brasil -carol_demarco@hotmail.com
IV Universidade
Federal de Pelotas, RS, Brasil -thaysafonso@hotmail.com
VII Universidade Federal de Pelotas, RS, Brasil
-mausq@hotmail.com
VIII Universidade Federal
de Pelotas, RS, Brasil -robsonandreazza@yahoo.com.br
ABSTRACT
The current moment requires the development of new technologies that can
provide alternatives to conventional treatment and that efficiently remove
pollutants that are difficult to treat. Activated carbon has been highlighted
as low cost material that can be used as adsorbents
for the removal of contaminants. Thus, the aim of the present study was to
analyse the relevant literature related to the production of activated carbon
for the treatment of water. For the study, there were found 4,182 relevant
studies in the database of the Web of Science and from these restrictions and
readings were obtained 27 articles. The information obtained was: i - temporal evolution of publications, ii - distribution
of articles by periodicals, iii - spatial distribution, iv - precursor
material, v - activation technology, vi - pollutants and vii - treatment
efficiency. Results show that the activated carbon produced from corn and
industrial ash residues are good adsorbents. Dyes, heavy metals and phenols
were the most studied pollutants, and had the higher treatment efficiency
values. The approach of the present study allows to identify the main points of
this new technology and it helps to support new researches and applications.
Keywords: Scientometry. Biomaterials. Wastewater. Waste.
RESUMO
O
momento atual requer o desenvolvimento de novas tecnologias que possam fornecer
alternativas ao tratamento convencional e que removam eficientemente poluentes
de difícil tratamento. Com o crescente interesse em materiais de baixo custo
que possam ser empregados como adsorventes para a remoção de contaminantes, o
carvão ativado tem se destacado. Deste modo, o objetivo do presente estudo foi
analisar a literatura relevante relacionada à produção de carvão ativado para o
tratamento de água. Para o estudo, foram encontrados 4.182 estudos relevantes
na base de dadosdo Web of Science e destes, a partir de restrições e leituras,
obtiveram-se 27 artigos. As informações obtidas foram: (i) evolução temporal
das publicações, (ii) distribuição dos artigos por periódicos, (iii)
distribuição espacial, (iv) material precursor, (v) tecnologia de ativação,
(vi) modelos ajustados, (vii) poluentes e (viii) eficiência de tratamento. Os
resultados demonstram que o carvão ativado produzido a partir de resíduos da
produção de milho e cinzas industriais apresenta-se como bons adsorventes. Os
corantes, metais pesados e fenóis foram os poluentes mais estudados e que
também possuem maiores valores de eficiência de tratamento. A abordagem do
presente estudo permite identificar os principais pontos desta nova tecnologia
de uma forma capaz de produz resultados que ajudarão a apoiar novas pesquisas e
aplicações.
Palavras-chave: Cientometria. Biomaterial. Águas
residuárias. Resíduos.
1 Introduction
In recent decades, water treatment and supply systems
have withstood high pressure from industrial segments and the growing
population. Water quality criteria may become unreachable for certain
structures and values. In recent years, under a scenario of high environmental
pollution, there has been a gradual interest in the use of activated charcoal
for water and wastewater treatment systems (WONG et al., 2018). Thus, activated
charcoal has great utility in pollution control.
However, the use of commercial activated charcoal
becomes costly for the removal of large-scale contaminants, making more
economical alternative materials and methods to be sought for its production
(PALLARÉS; GONZÁLEZ-CENCERRADO; ARAUZO, 2018). The studies seek to find low
cost and high efficiency carbonaceous materials, as well as to understand the
adsorptive processes of these new coals from their characterization and the
study to the fit of models (PRADEEP et al., 2016).
In this context, it is highlighted the studies with
proposals for the use of waste as a raw material to obtain coal, because in
this way, it is promoted the reuse of a solid waste. Solid waste processing for
the production of activated charcoal can reduce its management and disposal
problems, as well as reducing production costs (AKPA; NMEGBU, 2014).
Activated carbon has excelled in the processes of
adsorption, purification, filtration, deodorization and separation. Adsorption
provides some advantages over classic water and wastewater treatment methods
such as low waste generation, efficient substance removal, simplicity of
operation, easy metal recovery and the possibility of reuse of the adsorbent
(HETTIARACHCHI et al., 2016). The ability to remove a wide variety of compounds
in contaminated waters has increased the demand for activated charcoal in
recent years (CRINI; LICHTFOUSE, 2018). Activated charcoal applications are
numerous and its properties will depend on the reaction conditions,
carbonization method and activation employed and essentially on the nature of
the precursor material (KOSHELEVA; MITROPOULOS; KYZAS, 2018).
The present study aimed to analyze the relevant literature
related to the production of activated charcoal for water treatment through a
scientometric analysis. The study determined the temporal evolution of
publications, the distribution of articles by journals and country, the
activation technology, the main precursor materials used, the best-adjusted
kinetic and isothermal models, the pollutants removed and the efficiency of
treatment with the activated carbon produced.
2
Materials and Methods
Data were obtained from the Web of Science (WoS) database
for the years 1987-2017, totaling a 30-year search period. Data collection was
performed from four WoS research fields, each field being directed to a
research interest. Operators were employed to produce a detailed search and
generate subject queries with some variations. The proposed research fields
were: a) Object of interest of the study: activatedcharcoal$ OR activatedcarbon$;
b) Application of activated charcoal: watertreat* OR waterremov* OR
wateradsorpt* OR wateradsorbent*; c) Reusable or waste-activated activated
carbon: renew* coal$ OR reus* coal$ OR waste$; d) Cost of production and/or
obtaining: lowcostadsorbent$ OR lowcostcoal$.
The studies obtained for the initial search were 4,182
studies. After using some restrictions in the search field, a total of 27
articles were obtained. The search field selection and restriction procedures
are described in the flowchart proposed in Figure 1.
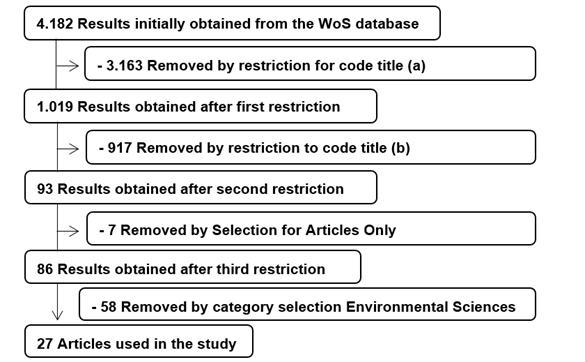
Figure 1 -
Flowchart of the data collection process from the inserted search field and
restrictions applied for the systematic review. Source: Authors.
The articles found were reevaluated for their
relevance to the topic of interest through readings of titles and abstracts.
Thus, some publications may have been withdrawn if they were not related to the
proposal of the work. From the final number of articles, after all screening
and restrictions, information was extracted from each article for data
collection. The extracted
information and its importance are described below:
a) Year: The analysis of the temporal distribution of
this study is given by collecting the years of publication;
b) Journal: The determination of the distribution of
articles by journal was determined according to the number of articles about
the published subject and the impact factor of this in the academic scope;
c) Country: The analysis of the spatial distribution
of this study is performed by collecting the countries of each publication. The
country chosen refers to the first author's address, and this one directly
relates to the research investment and his interest in the new technology. It
should be noted that there were works in which the precursor material is from a
different location from the country of authorship of the publication;
d) Raw material used: The precursor materials used for
the production of activated charcoal were collected from each publication to
identify the most used materials;
e) Temperature: The carbonization or pyrolysis process
consists of burning the raw material used. The study of the employed
temperature is very important to determine the amount of final carbon present
as well as the porous arrangement;
f) Activation technology: The activation process
consists of unclogging the pores and increasing the surface area. The
importance of this process is in its relationship with the adsorptive capacity
presented by coal and its surface functional groups. In the study, articles
were classified into chemical or physical activation;
g) Adjusted models: The behavior of the coals can be
understood by adjusting to kinetic and isothermal models. This process is of
great importance in designing a water and wastewater treatment system as well
as in achieving efficiency control throughout the process. The best fit kinetic
and adsorption isotherm models were collected;
h) Pollutant removed: The analysis of the main
pollutants removed by the activated charcoal produced sought to identify the
main contaminants, its problem in water treatment and their interaction with
the coal surface functional groups.
i) Treatment efficiency: Treatment efficiency and
production data were observed. When comparing the efficiency values the
amount of adsorbent added as well as its specific porosity must be taken into
account. However, it is important to highlight that many studies do not
characterize the material and do not describe the amounts of adsorbent used,
thus presenting only the initial and final pollutant concentration values and
the adjustment parameters of the models.
The results were analyzed by descriptive statistics
with the aid of RStudio software only for understanding their dispersion and
obtaining mean values, variances and deviations. The use of graphical analysis
demonstrates the trends, outliers and data organization for the study.
3
Results
and Discussion
Data obtained from the WoS database for the years 1987
to 2017 totaled a 30-year search period; however, all studies meeting the
search criteria were published after 2000 (Figure 2). The low occurrence of
studies in the past may be related to the low cost of obtaining and producing activated
charcoal as well as the low demand for its large-scale use. The results showed
a low relationship between the journals with the largest number of publications
on the subject and the impact factor values found, respectively.
A study conducted by MERCURI et al., (2016) found no
relationship between the impact factor and relative frequency of studies per
journal calculated for their area of study, thus demonstrating that the impact
factor should not be considered isolated.
The years of 2006 and 2008 did not present results for
the research. The largest number of studies found was conducted from 2013 to
2016, with 46.2% of the total number of articles found. The year of 2013 was
found with the largest number of studies on the subject. The largest number of
publications in the area were found for Environmental Technology Reviews and the
highest impact factor observed for Water Research. It is important to note that
for 2017 only articles published until April were considered. Further research
may be conducted later this year for further understanding of the study.
The spatial distribution of articles is concentrated
on the Asian continent (Figure 3). The countries with the most research in the
area were India and China respectively. Only these two countries accounted for
almost 50% of publications in the area.
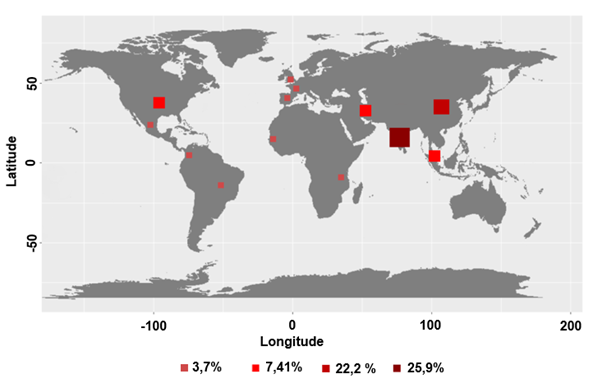
Figure
3 - Spatial distribution of studies. Source: Authors.
The results suggest a strong Asian interest in the use
of activated charcoal for water treatment. However, the analysis performed for
the application scale of the activated charcoal produced showed that all
studies performed were laboratory scale, and of these, only 7% presented
proposals for future large-scale applications. The result obtained for
application scale may be related to the lack of interest of large public and
private sector companies in the application of this technology. It is important
to highlight that the application scale is directly related to the cost of
obtaining and that it depends on the precursor material chosen. The precursor
materials for activated carbon production found in the present study can be seen
in Figure 4.
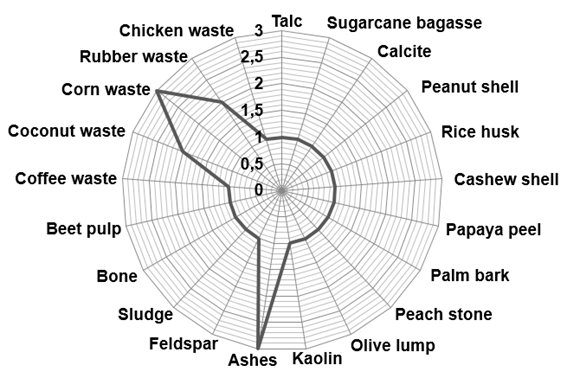
Figure 4 -
Raw material used in the activated carbon production process found for the
literature approached. Source: Authors.
The most
used materials in the coal production process were the ashes of the industrial
burning process and the residues from the corn production. Considering that the
carbonization process is essential for the production of activated charcoal
(TOVAR et al., 2019), the predominance of the use of materials from the burning
process was already expected. The results also suggest that large-scale
production processes, which consequently generate a large volume of waste, are
one of the criteria for choosing the precursor material.
The
choice of raw material becomes a determining factor for achieving high yield in
the activated carbon production process. Many materials have adsorption capacity, however, few materials perform this function with
high efficiency, causing the demand for new materials (GODIYA et al., 2019).
Thus, the nature of the precursor material, carbonization process temperature
and activation method are critical to achieving the expected efficiency.
In the
carbonization or pyrolysis process the precursor material is subjected to high
temperatures where volatile chemical compounds (H, N and O) and light gases (CO2,
H2, CO and CH4) are removed forming a fixed primary
porous carbon mass (PEDROZA et al., 2019). The study of the temperature
employed in this process is necessary to understand its properties and the
adsorptive process. In the analysis of the literature found, different
temperature values were observed for the precursor materials (Figure 5).
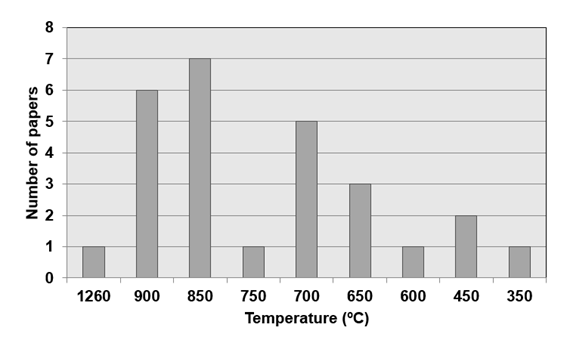
Figure
5 - Temperature values found in the carbonization process of the raw material
for the production of activated charcoal. Source: Authors.
It is
evident that light and moist materials require lower temperatures in the
process. The highest temperature found in the studies was 1260ºC for feldspar
material, and similarly, the lowest temperature recorded was 350ºC for residues
from corn production. The average
temperature value found was 765.2ºC.
In this
context, the burnt material is fundamentally microporous, but this porosity may
be filled or partially blocked by the decomposition and burning products. Therefore,
activation is required to unblock and enlarge the pores formed in this process
(CASTRO et al., 2018).
The
activation process aims to remove the organic compounds present such as tar,
creosote and naphtha, as well as other residues that may clog the pores. The
studies were classified according to the possible activation nature, which may
be chemical or physical (MAMANÍ et al., 2019). For the chemical activation
process there are 92.6% of the chosen works. The high use of the chemical
activation process in relation to the physical process may be related to ease
of operation, low process cost as well as high efficiency after application of
the method.
It is
important to remember that chemical activation is driven by the impregnation of
a dehydrating or oxidizing chemical on the precursor material, while physical
activation involves the exposure of activated carbon to vapors and
oxygen-containing gases (LIEW et al., 2018).
The
chemical and physical activation agents found in the literature are presented
in Figure 6.
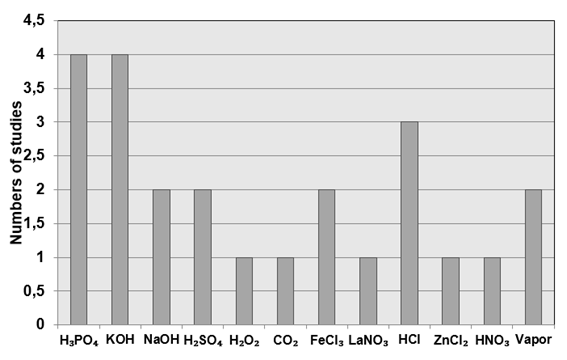
Figure
6 - Chemical and physical agents used in the activation process of the charcoal
produced. Source: Authors.
After the
production of activated carbon, it is necessary to study its characteristics
and adsorptive capacity. The use of methodologies to determine their
characteristics may be necessary. In the present study, it was decided to
determine the adsorptive capacity in relation to the adjustment of these coals
to kinetic and isothermal models. Methods of determining physical and chemical
characteristics may differ greatly and depend greatly on laboratory conditions
and experimental errors.
The study
of adsorption kinetics is essential because it provides information on the
speed at which reactions occur, the factors that influence adsorption, and the
interactions that occur at the adsorbate-adsorbent interface. Adsorption
kinetics also allow the determination of the amount of solute that is removed
from a solution over time, being of great importance in the design of an
effluent treatment system and also for obtaining process efficiency control
(KAUSAR et al., 2018). Adsorption isotherms, which take into consideration the
study of equilibrium, can provide important information for the evaluation of
affinity or adsorption capacity, being an excellent selection criterion. It can
determine the surface area of the adsorbent, the pore volume and the
statistical distribution as well as the determination of the optimum heat at
adsorption. Isotherms characterize the equilibrium of total solute
concentration over adsorbed concentration and solution concentration (KOOPAL;
TAN; AVENA, 2019).
The
models found for kinetic adjustment were Pseudo First Order, Pseudo Second
Order, Elovich and Interparticle Diffusion. To adjust the isotherms, the
equations of Langmuir, Freundlich, Tempkin and Dubinin – Radushkevich and Redlich-peterson
were found. The adjusted work numbers for each model are shown in Figure 7.
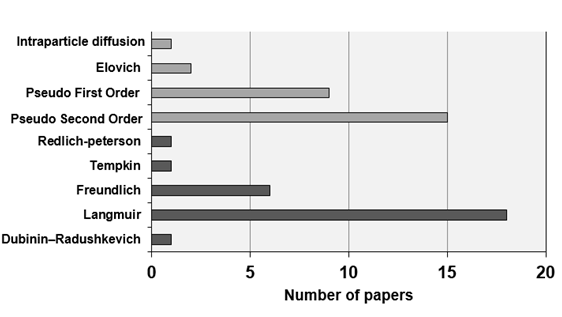
Figure
7 - Kinetic model and adsorption isotherm adjusted for each study conducted
with the activated carbon produced. Source: Authors.
The results show that the Pseudo First Order kinetic
model (55.6%) and the Langmuir equation (66.7%) were better adjusted to the
activated charcoal studies. This result implies the determination of the
adsorptive behavior of this material.
To understand the results, it is worth remembering
that the Pseudo First Order model assumes that adsorption occurs as a result of
a concentration gradient and the Langmuir equation suggests that every adsorbed
species interacts only with a defined site, each active site accommodates only
one molecule, there are no interactions between neighboring site molecules and
the adsorption energy of each site is equal (SONG et al., 2018). The models
fitted together suggest that the molecules are adsorbed due to concentration
gradient and that they adhere to the surface of activated carbon at
well-defined and localized active sites.
It is important to highlight the main difference
between the most widely used isotherms is that Freundlich does not predict
adsorbent saturation, unlike Langmuir. These two equations fit most systems
employed with activated charcoal, but Freundlich is supposed to better
represent systems with real effluents. The isotherm models of the Langmuir and
Freundlich equations are the most used.
The study of the adsorbable molecules is then
employed. Different toxic compounds difficult to remove by conventional
treatment are studied. The pollutants found can be observed in Figure 8.

Figure 8 -
Pollutant removed by treatment with activated charcoal produced from alternative
precursor material according to the literature. Source: Authors.
The main pollutants studied
were arsenic, dyes and phenol. The percentage of these studies was 41.7%. The
spatial distribution of the studies compared to the pollutant group
demonstrates a global interest in removing these pollutants, as studies in
South Africa, China, Spain, India, England, Iran, Mexico and Senegal were
conducted for this purpose.
Another important factor that
should be considered in the analysis of the pollutants studied is the temporal
variability. The group of the main compounds studied is present during the
research period, thus demonstrating that these are substances that are
difficult to treat and require studies with proven efficiency in the field.
The specificity between
adsorbate and adsorbent leads to the need to expand studies in the area. The
use of activated carbon adsorption as a method of water and wastewater
treatment has been of interest to different researchers (HAGEMANN et al.,
2018).
The efficiencies found by all
studies were gathered into similar or similarly toxic chemical compounds and
their averages were calculated. The efficiency classes are shown in Figure 9.
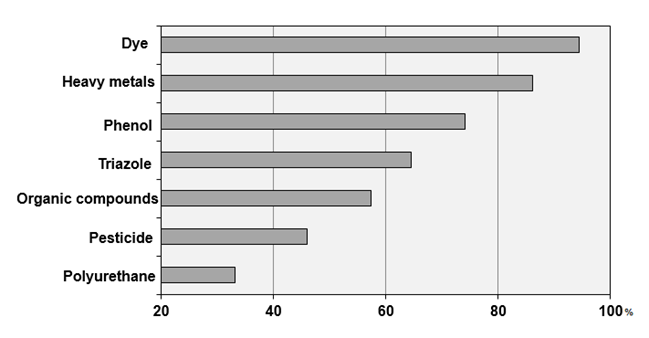
Figure 9 -
Pollutant removal efficiency. Source: Authors.
The results demonstrate a high
efficiency in the treatment of dyes and heavy metals. Most commercially used
dyes and metals are resistant to biodegradation, photodegradation and oxidizing
agent treatment processes. Proper treatment of these is necessary, because when
discharged without proper treatment in water bodies are responsible for serious
damage to biological systems and human health (AFROZE; SEN, 2018). The
adsorption of inorganic compounds by activated carbon has been widely studied,
and its purpose is to remove pollutant metals from liquid discharges and to
recover certain valuable metals.
Polyurethane treatment was the
least efficient treatment followed by pesticides and organic compounds
treatment. These may be related to pore arrangement, exposure time to
carbonization and activation processes, specific affinity between adsorbate and
adsorbent, molecule size and concentration of the pollutant under study.
Importantly, low-efficiency coals can be extremely microporous, while the
molecules of these pollutants are larger than the size of their pores. Dyes and
heavy metals are smaller molecules, and therefore easier to be adsorbed on
small sites compared to other types of pollutants.
Several studies corroborate
the results found. A study conducted by GOLIN (2007) describes in its review
that precursor materials such as tires, pits, wood scraps and ashes have good
removal efficiency of adsorbents such as cadmium, cobalt, copper, lead,
mercury, nickel and zinc. SOARES (1998) observed a removal efficiency of over
95% for the activated carbon monoclotriazine yellow and red by activated carbon
dyes from mining. CLAUDINO (2003) investigating the production of activated
charcoal from agricultural waste from southern Santa Catarina State found high
phenol removal.
The removal efficiency values
may vary according to different factors such as process time, pollutant
concentration, amount of adsorbent and pH. DA SILVA et al., (2016) found
removal efficiency between 76 and 93% for three different activated carbons.
The results demonstrate that
most of the precursor materials used were good adsorbents. Changes in
production processes such as variations in temperature and time of exposure to
burning, as well as the activating agent chosen may be responsible for the
increased adsorptive capacity. In
addition, modifications in the adsorption process such as pH and temperature
control, variation in agitator speed and form of column organization may be
responsible for the increase of efficiency.
4
Conclusions
The study showed that
activated charcoal is an important tool to control environmental pollution and
is even more employed when produced from waste. The precursor materials as well
as the carbonization and activation methods were relatively well suited for
most of the verified studies.
The results show that in the
last 30 years, the 2000 year has been an initial start in the production of papers
regarding alternative materials for the production of activated charcoal. It is
evident that other studies were produced before this year, but these did not
come under the desired initial conditions or were not in the Environmental
Sciences category. The spatial distribution of the articles showed that India
and China have a great interest in this subject.
The interest of the study in
promoting the knowledge of the main materials and pollutants studied, as well
as the production methods were addressed. The gaps are mainly related to the
limited number of large or medium scale studies. Many interesting studies with large-scale
application focus on commercial activated carbon. However, the increasing
number of publications on the subject in recent years may be an indication that
there is a scientific effort to promote the combination between laboratory
studies and the market.
The results showed that there
are a wide variety of precursor materials capable of becoming good adsorbents
and that their application in water treatment can be very successful. Activated
carbon produced from alternative materials presents good removal efficiency for
most of the studied compounds.
The study approach has
identified the main strengths and weaknesses of this new technology in a way
that can produce good results that will help support new research and
applications in this field from a global perspective.
Ackowledgments
This
study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de
Nível Superior - Brasil (CAPES) - Finance Code 001.
References
AFROZE S, SEN TK. A
review on heavy metal ions and dye adsorption from water by agricultural solid
waste adsorbents. Water, Air, & Soil Pollution. 2018; 229(7). doi:10.1007/s11270-018-3869-z
AKPA JG; NMEGBU CGJ. Adsorption of benzene on
activated carbon from agricultural waste materials research. Journal of
Chemical Sciences. 2014; 4(9): 34-40.
CASTRO JP, NOBRE JRC, BIANCHI ML, TRUGILHO PF, NAPOLI A,
CHIOU B-S, WILLIAMS TG, WOOD DF, AVENA-BUSTILLOS RJ, ORTS WJ, TONOLI GHD.
Activated carbons prepared by physical activation from different pretreatments
of amazon piassava fibers. Journal of Natural Fibers. 2018: 1-16.
CLAUDINO A. Preparação de
carvão ativado a partir de turfa e sua utilização na remoção de poluentes.
2003.
CRINI G, LICHTFOUSE
E. Advantages and disadvantages of techniques used for wastewater treatment. Environmental
Chemistry Letters. 2018; 17(1): 145-155.
DA SILVA FB, DO NASCIMENTO CW,
ARAUJO PR, DA SILVA LH,
DA SILVA RF. Assessing heavy metal sources in sugarcane Brazilian
soils: an approach using multivariate analysis. Environ Monit Assess. 2016; 188(8):
457.
FERREIRA MERCURI EG, JAKUBIAK
KUMATA AY, AMARAL EB, SIMÕES VITULE JR. Energy by Microbial Fuel Cells:
Scientometric global synthesis and challenges. Renewable and Sustainable Energy
Reviews. 2016; 65: 832-840.
GODIYA CB, CHENG X,
LI D, CHEN Z, LU X. Carboxymethyl cellulose/polyacrylamide composite hydrogel
for cascaded treatment/reuse of heavy metal ions in wastewater. J Hazard Mater;
2019; 364: 28-38.
GOLIN DM. Remoção de chumbo de meios líquidos
através de adsorção utilizando carvão ativado de origem vegetal e resíduos
vegetais. [dissertation]. Curitiba: Universidade Federal do Paraná, Programa de
Pós-Graduação em Engenharia de Recursos Hídricos e Minerais, 2007. 111 p.
HAGEMANN N, SPOKAS
K, SCHMIDT H-P, KÄGI R, BÖHLER M, BUCHELI T. activated carbon, biochar and
charcoal: linkages and synergies across pyrogenic carbon’s ABCs. Water. 2018; 10(2):182.
HETTIARACHCHI E,
PERERA R, CHANDANI PERERA ADL, KOTTEGODA N. Activated coconut coir for removal
of sodium and magnesium ions from saline water. Desalination and Water
Treatment. 2016; 57(47): 22341-22352.
KAUSAR A, IQBAL M,
JAVED A, AFTAB K, NAZLI ZIH, BHATTI HN, NOUREN S. Dyes adsorption using clay
and modified clay: A review. Journal of Molecular Liquids. 2018; 256: 395-407.
KOOPAL L, TAN W,
AVENA M. Mixed ad/desorption kinetics unraveled with the equilibrium adsorption
isotherm. Colloids and Surfaces A: Physicochemical and Engineering Aspects.
2019; 577: 709-722.
KOSHELEVA RI, MITROPOULOS AC, KYZAS GZ. Synthesis
of activated carbon from food waste. Environmental Chemistry Letters. 2018; 17(1):
429-438.
LIEW RK, CHONG MY,
OSAZUWA OU, NAM WL, PHANG XY, SU MH, CHENG CK, CHONG CT, LAM SS. Production of
activated carbon as catalyst support by microwave pyrolysis of palm kernel
shell: a comparative study of chemical versus physical activation. Research on
Chemical Intermediates. 2018; 44(6): 3849-3865.
MAMANÍ A, SARDELLA
MF, GIMÉNEZ M, DEIANA C. Highly microporous carbons from olive tree pruning:
Optimization of chemical activation conditions. Journal of Environmental
Chemical Engineering. 2019; 7(1): 102830.
PALLARÉS J,
GONZÁLEZ-CENCERRADO A, ARAUZO I. Production and characterization of activated
carbon from barley straw by physical activation with carbon dioxide and steam. Biomass
and Bioenergy. 2018; 115: 64-73.
PEDROZA MM, SILVA APOD, MELO JVD, PAZ
EDCS, PAZ RRDS. Ensaio de adsorção de ácido acético em carvão
produzido a partir da fibra de coccus nucifera l. Brazilian Journal
of Development,
2019; 5(6): 4784-4796.
PRADEEP GG,
SUKUMARAN KP, GEORGE G, MUHAMMAD F, MATHEW, N. Production and characterization
of activated carbon and its application in water purification. International
Research Journal of Engineering and Technology. 2016; 3(8).
SOARES JL. Remoção de corantes têxteis por adsorção em
carvão mineral ativado com alto teor de cinzas. 1998.
SONG T, YU C, HE X, LIN J, LIU Z, YANG X, ZHANG Y, HUANG Y, TANG C. Synthesis of
magnetically separable porous BN microrods@Fe3O4 nanocomposites for Pb(II)
adsorption. Colloids and Surfaces A: Physicochemical and Engineering Aspects.
2018; 537: 508-515.
TOVAR AK, GODINEZ
LA, ESPEJEL F, RAMIREZ-ZAMORA RM, ROBLES I. Optimization of the integral
valorization process for orange peel waste using a design of experiments
approach: Production of high-quality pectin and activated carbon. Waste Manag.
2019; 85: 202-213.
WONG S, NGADI N, INUWA IM, HASSAN O. Recent advances
in applications of activated carbon from biowaste for wastewater treatment: A
short review. Journal of Cleaner Production. 2018; 175: 361-375.